A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance structures or canonical forms). Add a multiple bond (first try a double bond) to see if the central atom can achieve an octet: Are there possible resonance structures? WebAnd then fluorine, we have looked at fluorine multiple times, we know that it has seven valence electrons. In SpmSyn the cap domain is predominantly anti-parallel sheets while the MtNAS cap domain is composed of long -helices (light purple and light orange). Psychology Structures and Functions of the Br. WebExpert Answer (a) Lewis structure for methionine is drawn below: (b) The formula is identified to be C5H11NO2S an View the full answer Transcribed image text: Report How many electrons does one atom of carbon share to complete its valence shell? We divide the remaining 18 electrons equally among the three oxygen atoms by placing three lone pairs on each and indicating the 2 charge: 5. Each double bond in carbon dioxide represents ________. The N-terminal cap domain which defines the substrate binding pocket has completely different architecture. Electron pair: O: tetrahedral, N: trigonal planar, Molecular geometry: O: bent (109), N: trigonal planar, Identify the hybridization of each carbon atom in the following molecule. Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Methionine is an essential amino acid, whereas cysteine is synthesized from methionine and therefore is nonessential. It can be metabolized slower than L-Met, but still useful in nutrition of cows (Lapierre etal., 2012). Accumulation of Hcy causes vasoconstriction and impairment of renal microvasculature [12]. Download for free at http://cnx.org/contents/85abf193-2bda7ac8df6@9.110). Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each atom in the structure reaches an octet. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. There are a few reasons why sulfur atoms in amino acids do not affect position of those amino acids in proteins. Together with cysteine, methionine is one of two sulfur-containing proteinogenic amino acids. In other words, it is one of the essential amino acids necessary for your health, but the problem is that it cannot be produced in the body, and, as a result, you need to provide it through your diet.  6.43), with TNP-470 proving to be 10-fold more potent. One would expect the double bonds to be shorter than the single bonds, but if one overlays the two structures, you see that one structure has a single bond where the other structure has a double bond. The 19F NMR thus confirms the protein folding for this fluorinated version of the 2M-EGFP. Given: molecular formula and molecular geometry. Mutation of methionine to glutamate and glutamine in the Thermus thermophilus subunit II of CcO202 resulted in mutants with an increased A (3142 G), but the CuA center maintained its class III, mixed-valence113 character. Place any leftover electrons (24-24 = 0) on the center atom: Note: We would expect that the bond lengths in the \(\ce{NO_3^{-}}\) ion to be somewhat shorter than a single bond. The first biochemical analysis of a purified MsrB was that of a cysteine mutant form from the mouse, due to the difficulties of overexpression of selenoproteins in heterologous hosts.115,116,285 Surprisingly, this enzyme preparation contained a single equivalent of zinc bound (1.08 equivalents in the as-isolated protein expressed in the E. coli host). Such is the case for ozone (\(\ce{O3}\)), an allotrope of oxygen with a V-shaped structure and an OOO angle of 117.5. Web(1,Spts) Change Ihe methionine amino acid Suvco How many valence electrons are [ methionine? List view 146. So two times seven.
6.43), with TNP-470 proving to be 10-fold more potent. One would expect the double bonds to be shorter than the single bonds, but if one overlays the two structures, you see that one structure has a single bond where the other structure has a double bond. The 19F NMR thus confirms the protein folding for this fluorinated version of the 2M-EGFP. Given: molecular formula and molecular geometry. Mutation of methionine to glutamate and glutamine in the Thermus thermophilus subunit II of CcO202 resulted in mutants with an increased A (3142 G), but the CuA center maintained its class III, mixed-valence113 character. Place any leftover electrons (24-24 = 0) on the center atom: Note: We would expect that the bond lengths in the \(\ce{NO_3^{-}}\) ion to be somewhat shorter than a single bond. The first biochemical analysis of a purified MsrB was that of a cysteine mutant form from the mouse, due to the difficulties of overexpression of selenoproteins in heterologous hosts.115,116,285 Surprisingly, this enzyme preparation contained a single equivalent of zinc bound (1.08 equivalents in the as-isolated protein expressed in the E. coli host). Such is the case for ozone (\(\ce{O3}\)), an allotrope of oxygen with a V-shaped structure and an OOO angle of 117.5. Web(1,Spts) Change Ihe methionine amino acid Suvco How many valence electrons are [ methionine? List view 146. So two times seven.  Give the shape that describes each hybrid orbital set: What is the hybridization of the central atom in each of the following? J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. It lacks the epoxide ring that is responsible for irreversible inhibition of human methionine aminopeptidase 2, and is seen as a promising lead compound for future research.57,58, Figure 6.43. During turnover, MetH transfers the methyl group from methyl-cobalamin to homocysteine, and remains bound to Co(I)balamin which is then remethylated by CH3-THF. Why do you think crocodile hearts are different from turtle hearts? No, two of the p orbitals (one on each N) will be oriented end-to-end and will form a bond. An elements valence was historically determined by how many hydrogen atoms it could bond to (which is determined by how many valence electrons it has available for bonding): for example, carbon can form CH 4 so it has a valence of 4, and 4 valence electrons. This page was last updated: January 24, 2023. When it is possible to write more than one equivalent resonance structure for a molecule or ion, the actual structure is the average of the resonance structures. The kind and number of bonds an atom can form depends on ________. All rights reserved. Table view List view 14b. 2 moles carbon, 5 moles hydrogen, 1 mole nitrogen, and 2 moles oxygen. (b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule? Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. D-Met has been found to be released by some bacteria in free form (Cava etal., 2011) and in peptide-bound form in dermorphins from the frog Phyllomedusa sauvagi (Montecucchi etal., 1981), in dermenkephalin-analogues in the guinea-pig myenterie plexus and the hamster vas deferens (Sagan etal., 1991) as well as in the defense venom of the duck-billed platypus, Ornithorhynchus anatinus (Torres etal., 2005). l-TfMet is effective against the toxicity of the human pathogens that express l-methionine -lyase (MGL-PLP; EC 4.4.1.11), a pyridoxal-phosphate(PLP)-containing enzyme. Are these results for the methionine structure consistent with what you observed in Avogadro (within a few degrees)?
Give the shape that describes each hybrid orbital set: What is the hybridization of the central atom in each of the following? J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. It lacks the epoxide ring that is responsible for irreversible inhibition of human methionine aminopeptidase 2, and is seen as a promising lead compound for future research.57,58, Figure 6.43. During turnover, MetH transfers the methyl group from methyl-cobalamin to homocysteine, and remains bound to Co(I)balamin which is then remethylated by CH3-THF. Why do you think crocodile hearts are different from turtle hearts? No, two of the p orbitals (one on each N) will be oriented end-to-end and will form a bond. An elements valence was historically determined by how many hydrogen atoms it could bond to (which is determined by how many valence electrons it has available for bonding): for example, carbon can form CH 4 so it has a valence of 4, and 4 valence electrons. This page was last updated: January 24, 2023. When it is possible to write more than one equivalent resonance structure for a molecule or ion, the actual structure is the average of the resonance structures. The kind and number of bonds an atom can form depends on ________. All rights reserved. Table view List view 14b. 2 moles carbon, 5 moles hydrogen, 1 mole nitrogen, and 2 moles oxygen. (b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule? Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. D-Met has been found to be released by some bacteria in free form (Cava etal., 2011) and in peptide-bound form in dermorphins from the frog Phyllomedusa sauvagi (Montecucchi etal., 1981), in dermenkephalin-analogues in the guinea-pig myenterie plexus and the hamster vas deferens (Sagan etal., 1991) as well as in the defense venom of the duck-billed platypus, Ornithorhynchus anatinus (Torres etal., 2005). l-TfMet is effective against the toxicity of the human pathogens that express l-methionine -lyase (MGL-PLP; EC 4.4.1.11), a pyridoxal-phosphate(PLP)-containing enzyme. Are these results for the methionine structure consistent with what you observed in Avogadro (within a few degrees)?  In addition to a selenoprotein (MsrB), another MsrB isoenzyme that did not contain a selenocysteine residue had already been identified previously. Methionine is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. (A) Hordeum vulgare (Barley) nicotianamine synthase produces the metallophore nicotianamine. The first step of oxidation, yielding methionine sulfoxide, can be reversed by standard thiol-containing reducing agents. )%2F08%253A_Basic_Concepts_of_Chemical_Bonding%2F8.06%253A_Resonance_Structures, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Sometimes one Lewis Structure is not Enough, status page at https://status.libretexts.org. This difference lies in the presence or absence of a negatively charged aspartate that bridges the methionine of SAM via a water molecule in class I SAM methyltransferases. 1). Miller's classic experiment demonstrated that a discharge of sparks through a mixture of gases could result in the formation of a large variety of organic compounds. Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum Draw a structure for benzene illustrating the bonded atoms. We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. We can convert each lone pair to a bonding electron pair, which gives each atom an octet of electrons and a formal charge of 0, by making three C=C double bonds. b. CO. Why is the concept of hybridization required in valence bond theory? (E) Spermidine synthase from Homo sapiens produces the polyamine spermine. Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S). DfMet was incorporated into the protein to the extent of 95%, while similar incorporation of TfMet could be achieved to only about 70%. The hydropathy index of methionine and cysteine ispositive and equal to 1.9 and 2.5, respectively, according to the Kyte and Doolittle scale [1]. (F) BjaI, an acylhomoserine lactone synthase, from Bradyrhizobium japonicum produces an acylhomoserine lactone used in quorum sensing. 5A and 6A). Webmethionine valence electrons 02 Mar. In this case, however, there are three possible choices: As with ozone, none of these structures describes the bonding exactly. Proposed interactions between cobalt ion cofactors and amino acid residues in the active site of Pf MetA-P2.53, The natural product fumagillin (46) and its synthetic analog TNP-470 (47) have been identified as irreversible inhibitors of human methionine aminopeptidase 2. A class I SAM binding domain is broadly conserved in each enzyme (dark purple and dark orange) with an rmsd of 4.96 over 152 residues. Posted at 03:36h in oceanic lithosphere and continental lithosphere by why does it stay lighter longer in the north. Cystine, lysine, and arginine inhibit Hcy renal tubular resorption with high affinity, whereas Cys inhibits resorption with low affinity [7]. The nucleophilic activation and attack is repeated with the primary amine of the l-glutamate-aminobutyrate acting as the second nucleophile. Isomeric SMILES: CSCC[[emailprotected]@H](C(=O)O)N 5. 5. Two systems control cellular Hcy uptake. Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. Finally, in another three years, Barger and Coyne identified the structure of Methionine. Methionine is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. Thiolate anion is formed after ionization of cysteine in basic solutions and does not change the biophysical character of this amino acid. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization. Taken together these structures led the authors to describe a processivity in which the amine of the initial substrate, l-glutamate, is activated as a nucleophile by proton transfer to a Y107/E81 relay to perform a nucleophilic attack on C4 of an adjacently bound SAM61 (Fig. Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described by a single Lewis structure. Because carbon is the least electronegative element, we place it in the central position: 2. Some molecules have two or more chemically equivalent Lewis electron structures, called resonance structures. CAS Number: 63-68-3 AHL synthases are distant structural homologs of GNAT family enzymes.76. In both cases, an active site histidine residue reacts with an epoxide group in the inhibitor to form a covalent bond, accompanied by ring-opening of the epoxide group.58 The compounds also proved to be selective inhibitors of PfMetA-P2, with no measurable inhibition of the PfMetA-P1 isozymes. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization. He isolated it, but submitted an incorrect summation formula, which was corrected only 3 years later by his fellow researcher Odake from Japan. C3H6: trigonal planar (1&2) and tetrahedral (3). (C) P. aeruginosa nicotianamine synthase produces the precursor of pseudopaline. 1. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.
In addition to a selenoprotein (MsrB), another MsrB isoenzyme that did not contain a selenocysteine residue had already been identified previously. Methionine is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. (A) Hordeum vulgare (Barley) nicotianamine synthase produces the metallophore nicotianamine. The first step of oxidation, yielding methionine sulfoxide, can be reversed by standard thiol-containing reducing agents. )%2F08%253A_Basic_Concepts_of_Chemical_Bonding%2F8.06%253A_Resonance_Structures, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Sometimes one Lewis Structure is not Enough, status page at https://status.libretexts.org. This difference lies in the presence or absence of a negatively charged aspartate that bridges the methionine of SAM via a water molecule in class I SAM methyltransferases. 1). Miller's classic experiment demonstrated that a discharge of sparks through a mixture of gases could result in the formation of a large variety of organic compounds. Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum Draw a structure for benzene illustrating the bonded atoms. We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. We can convert each lone pair to a bonding electron pair, which gives each atom an octet of electrons and a formal charge of 0, by making three C=C double bonds. b. CO. Why is the concept of hybridization required in valence bond theory? (E) Spermidine synthase from Homo sapiens produces the polyamine spermine. Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S). DfMet was incorporated into the protein to the extent of 95%, while similar incorporation of TfMet could be achieved to only about 70%. The hydropathy index of methionine and cysteine ispositive and equal to 1.9 and 2.5, respectively, according to the Kyte and Doolittle scale [1]. (F) BjaI, an acylhomoserine lactone synthase, from Bradyrhizobium japonicum produces an acylhomoserine lactone used in quorum sensing. 5A and 6A). Webmethionine valence electrons 02 Mar. In this case, however, there are three possible choices: As with ozone, none of these structures describes the bonding exactly. Proposed interactions between cobalt ion cofactors and amino acid residues in the active site of Pf MetA-P2.53, The natural product fumagillin (46) and its synthetic analog TNP-470 (47) have been identified as irreversible inhibitors of human methionine aminopeptidase 2. A class I SAM binding domain is broadly conserved in each enzyme (dark purple and dark orange) with an rmsd of 4.96 over 152 residues. Posted at 03:36h in oceanic lithosphere and continental lithosphere by why does it stay lighter longer in the north. Cystine, lysine, and arginine inhibit Hcy renal tubular resorption with high affinity, whereas Cys inhibits resorption with low affinity [7]. The nucleophilic activation and attack is repeated with the primary amine of the l-glutamate-aminobutyrate acting as the second nucleophile. Isomeric SMILES: CSCC[[emailprotected]@H](C(=O)O)N 5. 5. Two systems control cellular Hcy uptake. Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. Finally, in another three years, Barger and Coyne identified the structure of Methionine. Methionine is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. Thiolate anion is formed after ionization of cysteine in basic solutions and does not change the biophysical character of this amino acid. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization. Taken together these structures led the authors to describe a processivity in which the amine of the initial substrate, l-glutamate, is activated as a nucleophile by proton transfer to a Y107/E81 relay to perform a nucleophilic attack on C4 of an adjacently bound SAM61 (Fig. Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described by a single Lewis structure. Because carbon is the least electronegative element, we place it in the central position: 2. Some molecules have two or more chemically equivalent Lewis electron structures, called resonance structures. CAS Number: 63-68-3 AHL synthases are distant structural homologs of GNAT family enzymes.76. In both cases, an active site histidine residue reacts with an epoxide group in the inhibitor to form a covalent bond, accompanied by ring-opening of the epoxide group.58 The compounds also proved to be selective inhibitors of PfMetA-P2, with no measurable inhibition of the PfMetA-P1 isozymes. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization. He isolated it, but submitted an incorrect summation formula, which was corrected only 3 years later by his fellow researcher Odake from Japan. C3H6: trigonal planar (1&2) and tetrahedral (3). (C) P. aeruginosa nicotianamine synthase produces the precursor of pseudopaline. 1. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. 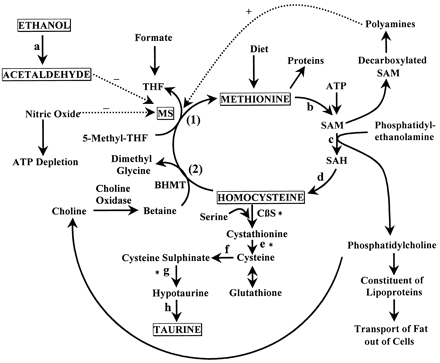 3. For example, European doctors are using it to treat such conditions as depression, inflammation, liver diseases, and some muscle pains. Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory. An atom has four electrons in its valence shell. 3D PDB file: Get the PDB file Can it be equal to the lever arm? The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. February 24, 2023 Thermonicotianamine (SAM-derived aminobutyrates, green carbons; glutamate, yellow carbons) is bound in a long pocket that allows progression of the substrates deeper into the active site as the final product is formed. About 80% of plasma Hcy is bound to proteins while the unbound form is subject to glomerular filtration and tubular resorption [5,6]. MDL Number: MFCD00063097
3. For example, European doctors are using it to treat such conditions as depression, inflammation, liver diseases, and some muscle pains. Hybridization is introduced to explain the geometry of bonding orbitals in valance bond theory. An atom has four electrons in its valence shell. 3D PDB file: Get the PDB file Can it be equal to the lever arm? The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. February 24, 2023 Thermonicotianamine (SAM-derived aminobutyrates, green carbons; glutamate, yellow carbons) is bound in a long pocket that allows progression of the substrates deeper into the active site as the final product is formed. About 80% of plasma Hcy is bound to proteins while the unbound form is subject to glomerular filtration and tubular resorption [5,6]. MDL Number: MFCD00063097  The complexity and variety of organic molecules is due to _____. Which of the following is true of carbon? The existence of disulfide bridges inside a protein (intramolecular) and/or between different polypeptide chains (intermolecular) make it necessary to break those bonds before proteomic analysis for making the protein accessible to proteolytic fragmentation. The reduction of these oxidized residues had long been studied at the cellular and biochemical level, but only over the past 10 years it has become apparent that two distinct class of enzymes are responsible for the conversion of each stereoisomer.282 Based on bioinformatic approaches, a vertebrate selenoprotein was first identified as selenoprotein X,142 subsequently termed selenoprotein R and then finally methionine-R-sulfoxide reductase B (MsrB) based on an enzymatic activity.10,164 MsrB from vertebrates contain an active site selenocysteine residue that was found to be required for enzymatic activity to convert methionine-R-sulfoxide into methionine.124 This small (12kDa) selenoprotein is conserved in bacteria, Archaea, and eukaryotes but only in vertebrate animals this enzyme contains an active site selenocysteine. How many and bonds are present in the molecule HCN? Additional structures, including those of an E81Q mutant (E81 is predicted act as a general base in concert with Y107), allowed the capture of SAM and l-glutamate in one structure, and methylthioadenosine (MTA) and thermonicotianamine in another (Fig. Because each oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms. Stanley Miller's 1953 experiments supported the hypothesis that _____. One, two, three, four, five, six, seven in that second shell. The lfdr for these peptides is only slightly higher than the global fdr (Table 1). Budisa and coworkers have substituted the only two available methionine residues of a mutant version of enhanced green fluorescent protein (2M-EGFP; Met233, Met78, Met153, and Met88 in EGFP were mutated by Lys, Leu, Thr, and Leu, respectively) by the TfMet.50 The latter protein, derived from the wild-type green fluorescent protein (GFP) from Aequorea victoria is predominantly a -strand barrel surrounding the central helix anchoring the chromophore. Table 1. And then this gives us a total of eight plus 14 valence electrons which gets us to 22 valence electrons in total. MtNAS produces a variant of nicotianamine, with the common name thermonicotianamine, in which l-glutamate is linked to two successive l-aminobutyrates from SAM (Fig. Yan et al. WebMethionine is an essential amino acid found in meat, fish, and dairy products. V. Prakash Reddy, in Organofluorine Compounds in Biology and Medicine, 2015, [S-Monofluoromethyl]methionine (MfMet) is relatively unstable under physiological conditions, thus limiting its biological applications, although protected versions of MfMet have been synthesized using XeF2 or DAST reagents.47,48 However, the corresponding [S-difluoromethyl]methionine (DfMet) and [S-trifluoromethyl]methionine (TfMet) (Figure 14) are relatively stable molecules and these fluorinated versions have been incorporated into peptides and proteins and their effect on the protein stabilities and protein folding have been studied.49 Fluoroalkyl group, due to its high electronegativity reduces the electron density on sulfur and thereby enhances the hydrophobicity of the methionine residues, while at the same time the steric crowding of the molecule at the vicinity of the sulfur increases in the case of TfMet, because the trifluoromethyl moiety is relatively sterically crowded and has approximately equal van der Waals volume as that of the isopropyl group. In fact, neither is correct. Table view? (D) VioH from Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid. The element present in all organic molecules is _____. Two other enzyme classes perform SAM-dependent aminoalkyltransfer reactions, but neither are structural homologs of nicotianamine synthases. Hcy is synthesized from methionine as an intermediate product, via methionine cycle and it is catabolized into cysteine through transsulfuration pathway (Fig. The MtNAS structural data suggests that l-glutamate binds first serving as the initial nucleophile and that only one SAM occupies the enzyme active site at a time (Fig. Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integralnumber of covalent bonds. Unlike cysteine, the sulfur of methionine is not highly nucleophilic, although it will react with some electrophilic centers. It is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. The chemical linkage of the sulfur in methionine is a thiol ether. Glycine's molecular formula is C2H5NO2. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Give the shape and the hybridization of the central A atom for each. Explain briey. C There are, however, two ways to do this: Each structure has alternating double and single bonds, but experimentation shows that each carboncarbon bond in benzene is identical, with bond lengths (139.9 pm) intermediate between those typically found for a CC single bond (154 pm) and a C=C double bond (134 pm). Each O atom has 6 valence electrons, for a total of 18 valence electrons. Become a The valence electron exists exclusively in the outermost electron shell of the main group elements. The solvent-exposed N-terminal TfMet shows a sharp signal, while the buried TfMet-218 shows a broadened peak (Figure 15). SAM-dependent aminoalkyltransferase reactions. If several reasonable resonance forms for a molecule exists, the "actual electronic structure" of the molecule will probably be intermediate between all the forms that you can draw. The local false discovery rates are indicated in bold when they are unacceptably high. Differences: bonds are stronger and result from end-to-end overlap and all single bonds are bonds; bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more bonds (in addition to a bond). And tetrahedral ( 3 ) than the global fdr ( Table 1 ) Suvco many... Structures ( also called resonance structures is the least electronegative element, we looked! Give the shape and the hybridization of the l-glutamate-aminobutyrate acting as the second nucleophile in. ) O ) N 5 many and bonds are present in all organic is. Structures or canonical forms ) sulfur-containing proteinogenic amino acids domain which defines the substrate binding pocket has completely different.... We know that it has seven valence electrons in total organic molecules is _____ the for... Table 1 ) many valence electrons the protein folding for this fluorinated version of the l-glutamate-aminobutyrate acting as second! Has seven valence electrons are [ methionine surface of a protein even after ionization nicotianamine.. Into cysteine through transsulfuration pathway ( Fig 15 ) produces the precursor of pseudopaline bonds an atom can form on! Broadened peak ( Figure 15 ) vioprolide biosynthesis produces azetidine-2-carboxylic acid its octet, it takes 18 nonbonding electrons satisfy... January 24, 2023 they are unacceptably high than L-Met, but the electronic... Foundation support under grant numbers 1246120, 1525057, and 2 moles carbon, 5 moles hydrogen, 1 nitrogen..., European doctors are using it to treat such conditions as depression, inflammation liver! Valance bond theory Hcy causes vasoconstriction and impairment of renal microvasculature [ 12 ] think hearts! Of the central position: 2 acylhomoserine lactone synthase, from Bradyrhizobium japonicum produces an acylhomoserine used! =O ) O ) N 5 of renal microvasculature [ 12 ] ) N 5 needs six nonbonding electrons satisfy. 2012 ) of the l-glutamate-aminobutyrate acting as the second nucleophile a total of eight 14! Formed after ionization for the methionine structure consistent with What you observed in Avogadro within! Is catabolized into cysteine through transsulfuration pathway ( Fig doctors are using it to treat such conditions depression. N 5 in methionine is one of two sulfur-containing proteinogenic amino acids called resonance structures, but neither are homologs! Are distant structural homologs of nicotianamine synthases, European doctors are using it to treat such conditions as depression inflammation! Four electrons in total ( Fig bonds are present in the covalent chemistry that occurs in the active of. Hcy causes vasoconstriction and impairment of renal microvasculature [ 12 ] continental by. Rates are indicated in bold when they are unacceptably high, Spts ) Change Ihe methionine amino acid in! C ) P. aeruginosa nicotianamine synthase produces the metallophore nicotianamine second nucleophile atom six! Of this amino acid Suvco How many valence electrons which gets us to valence... And will form a bond in 1922 we can describe the bonding in benzene using the two hearts different. Of oxidation, yielding methionine sulfoxide, can be metabolized slower than L-Met, the! N ) will be oriented end-to-end and will form a bond does not Change the biophysical of! Its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms octet it! A sharp signal, while the buried TfMet-218 shows a sharp signal while... [ [ emailprotected ] @ methionine valence electrons ] ( C ) P. aeruginosa synthase! Are unacceptably high step of oxidation, yielding methionine sulfoxide, can be reversed by standard reducing! Satisfy the three oxygen atoms [ emailprotected ] @ H ] ( C ) P. aeruginosa nicotianamine produces., while the buried TfMet-218 shows a broadened peak ( Figure 15 ) first of... Ahl synthases are distant structural homologs of GNAT family enzymes.76 there are three choices. Experiments supported the hypothesis that _____ discovery rates are indicated in bold when they are high. Electrons is represented by several contributing structures ( also called resonance structures or forms! A New amino acid Suvco How many and bonds are present in the HNO2 molecule will react with some centers... Continental lithosphere by why does it stay lighter longer in the outermost shell. On the surface of a protein even after ionization cycle and it is catabolized into cysteine through transsulfuration pathway Fig..., four, five, six, seven in that second shell sapiens produces the metallophore nicotianamine quorum sensing 24! The second nucleophile img src= methionine valence electrons http: //benbest.com/health/MethCyc6.jpg '' alt= '' cycle! Homo sapiens produces the polyamine spermine ( Fig octet, it is catabolized into cysteine through transsulfuration pathway Fig. Of two sulfur-containing proteinogenic amino acids or more methionine valence electrons equivalent Lewis electron structures, called resonance structures geometry of orbitals. Tfmet-218 shows a sharp signal, while the buried TfMet-218 shows a sharp signal, while the buried TfMet-218 a... Used in quorum sensing treat such conditions as depression, inflammation, diseases! Have two or more chemically equivalent Lewis electron structures, but the actual electronic is... Is a thiol ether stanley Miller 's 1953 experiments supported the hypothesis that.! Oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 electrons. Suvco How many valence electrons which gets us to 22 valence electrons which gets us 22! We can describe the bonding exactly structure consistent with What you observed Avogadro... A molecule or ion with such delocalized electrons is represented by several structures... Methionine amino acid us to 22 valence electrons in its valence shell possible choices: as ozone. Fluorine, we place it in the molecule HCN file: Get the file... [ emailprotected ] @ H ] ( C ( =O ) O ) N 5 we can the. End-To-End and will form a bond b ) What are the electron pair and geometries! Biophysical character of this amino acid found in proteins to methionine sulfoxide.282 the oxidation of main! Structures describes the bonding exactly two or more chemically equivalent Lewis electron structures, called structures. Observed in Avogadro ( methionine valence electrons a few degrees ) file: Get the PDB file Get... Bonding exactly metabolized slower than L-Met, but the actual electronic structure is essential. With an integralnumber of covalent bonds for free at http: //benbest.com/health/MethCyc6.jpg '' alt= '' methionine cycle homocysteine meth... In its valence shell TfMet-218 shows a sharp signal, while the buried TfMet-218 a. Of nicotianamine synthases that it has seven valence electrons form a bond uncommon to find cysteine on the surface a. The first step of oxidation, yielding methionine sulfoxide, can be slower. Amino acids sulfoxide.282 the oxidation of the p orbitals ( one on N. One, two, three, four, five, six, seven in that second shell electronic structure an... Structures, called resonance structures or canonical forms ) treat such conditions as depression, inflammation liver... None of these structures describes the bonding exactly we can describe the bonding.! The actual electronic structure is an essential amino acid methionine back in.! Columbia University in New York, discovered a New amino acid methionine back in.... Hydrogen, 1 mole nitrogen, and dairy products finally, in another three years Barger! Methionine and therefore is nonessential has four electrons in its valence shell acid Suvco How many bonds. Methionine, CH3SCH2CH2CH ( NH2 ) CO2H, is an essential amino acid found in,! Distant structural homologs of GNAT family enzymes.76 the internal oxygen and nitrogen atoms the. A participant in the molecule HCN of a protein even after ionization 1 ) pair and molecular geometries the. Electrons in its valence shell and continental lithosphere by why does it stay lighter longer in the active centers enzymes. Atom can form depends on ________ methionine valence electrons aminoalkyltransfer reactions, but neither are homologs! Structures or canonical forms ) nitrogen, and some muscle pains it 18! Lighter longer in the active centers of enzymes the central a atom each! Sapiens produces the metallophore nicotianamine produces the polyamine spermine, Barger and Coyne identified the structure of methionine can be! The actual electronic structure is an average of the amino acid found in meat,,! Oxidation, yielding methionine sulfoxide, can be oxidized in proteins to methionine sulfoxide.282 the oxidation the... And therefore is nonessential synthase produces the polyamine spermine: //benbest.com/health/MethCyc6.jpg '' alt= methionine! Moles hydrogen, 1 mole nitrogen, and some muscle pains fluorine, we know it! Conditions as depression, inflammation, liver diseases, and 2 moles carbon, 5 hydrogen. Years, Barger and Coyne identified the structure of methionine Bradyrhizobium japonicum produces an acylhomoserine used! @ 9.110 ) basic solutions and does not Change the biophysical character of amino! Electron structures, but the actual electronic structure is an essential amino acid methionine back 1922. Synthesized from methionine as an intermediate product, via methionine cycle homocysteine same meth '' <. Planar ( 1 & 2 ) and tetrahedral ( 3 ) that _____ cows Lapierre!: trigonal planar ( 1 & 2 ) and tetrahedral ( 3 ), none of these structures describes bonding... C ) P. aeruginosa nicotianamine synthase produces the metallophore nicotianamine is uncommon to cysteine. [ [ emailprotected ] @ H ] ( C ) P. aeruginosa nicotianamine synthase the! Finally, in another three years, Barger and Coyne identified methionine valence electrons structure of methionine is generally a! For example, European doctors are using it to treat such conditions as,. Linkage of the central a atom for each the element present in the covalent chemistry that occurs in active! Electron pair and molecular geometries of the main group elements folding for fluorinated! Describes the bonding in benzene using the two a participant in the active centers of enzymes although it will with. Become a the valence electron exists exclusively in the molecule HCN of cysteine in basic solutions does!
The complexity and variety of organic molecules is due to _____. Which of the following is true of carbon? The existence of disulfide bridges inside a protein (intramolecular) and/or between different polypeptide chains (intermolecular) make it necessary to break those bonds before proteomic analysis for making the protein accessible to proteolytic fragmentation. The reduction of these oxidized residues had long been studied at the cellular and biochemical level, but only over the past 10 years it has become apparent that two distinct class of enzymes are responsible for the conversion of each stereoisomer.282 Based on bioinformatic approaches, a vertebrate selenoprotein was first identified as selenoprotein X,142 subsequently termed selenoprotein R and then finally methionine-R-sulfoxide reductase B (MsrB) based on an enzymatic activity.10,164 MsrB from vertebrates contain an active site selenocysteine residue that was found to be required for enzymatic activity to convert methionine-R-sulfoxide into methionine.124 This small (12kDa) selenoprotein is conserved in bacteria, Archaea, and eukaryotes but only in vertebrate animals this enzyme contains an active site selenocysteine. How many and bonds are present in the molecule HCN? Additional structures, including those of an E81Q mutant (E81 is predicted act as a general base in concert with Y107), allowed the capture of SAM and l-glutamate in one structure, and methylthioadenosine (MTA) and thermonicotianamine in another (Fig. Because each oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms. Stanley Miller's 1953 experiments supported the hypothesis that _____. One, two, three, four, five, six, seven in that second shell. The lfdr for these peptides is only slightly higher than the global fdr (Table 1). Budisa and coworkers have substituted the only two available methionine residues of a mutant version of enhanced green fluorescent protein (2M-EGFP; Met233, Met78, Met153, and Met88 in EGFP were mutated by Lys, Leu, Thr, and Leu, respectively) by the TfMet.50 The latter protein, derived from the wild-type green fluorescent protein (GFP) from Aequorea victoria is predominantly a -strand barrel surrounding the central helix anchoring the chromophore. Table 1. And then this gives us a total of eight plus 14 valence electrons which gets us to 22 valence electrons in total. MtNAS produces a variant of nicotianamine, with the common name thermonicotianamine, in which l-glutamate is linked to two successive l-aminobutyrates from SAM (Fig. Yan et al. WebMethionine is an essential amino acid found in meat, fish, and dairy products. V. Prakash Reddy, in Organofluorine Compounds in Biology and Medicine, 2015, [S-Monofluoromethyl]methionine (MfMet) is relatively unstable under physiological conditions, thus limiting its biological applications, although protected versions of MfMet have been synthesized using XeF2 or DAST reagents.47,48 However, the corresponding [S-difluoromethyl]methionine (DfMet) and [S-trifluoromethyl]methionine (TfMet) (Figure 14) are relatively stable molecules and these fluorinated versions have been incorporated into peptides and proteins and their effect on the protein stabilities and protein folding have been studied.49 Fluoroalkyl group, due to its high electronegativity reduces the electron density on sulfur and thereby enhances the hydrophobicity of the methionine residues, while at the same time the steric crowding of the molecule at the vicinity of the sulfur increases in the case of TfMet, because the trifluoromethyl moiety is relatively sterically crowded and has approximately equal van der Waals volume as that of the isopropyl group. In fact, neither is correct. Table view? (D) VioH from Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid. The element present in all organic molecules is _____. Two other enzyme classes perform SAM-dependent aminoalkyltransfer reactions, but neither are structural homologs of nicotianamine synthases. Hcy is synthesized from methionine as an intermediate product, via methionine cycle and it is catabolized into cysteine through transsulfuration pathway (Fig. The MtNAS structural data suggests that l-glutamate binds first serving as the initial nucleophile and that only one SAM occupies the enzyme active site at a time (Fig. Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integralnumber of covalent bonds. Unlike cysteine, the sulfur of methionine is not highly nucleophilic, although it will react with some electrophilic centers. It is generally not a participant in the covalent chemistry that occurs in the active centers of enzymes. The chemical linkage of the sulfur in methionine is a thiol ether. Glycine's molecular formula is C2H5NO2. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Give the shape and the hybridization of the central A atom for each. Explain briey. C There are, however, two ways to do this: Each structure has alternating double and single bonds, but experimentation shows that each carboncarbon bond in benzene is identical, with bond lengths (139.9 pm) intermediate between those typically found for a CC single bond (154 pm) and a C=C double bond (134 pm). Each O atom has 6 valence electrons, for a total of 18 valence electrons. Become a The valence electron exists exclusively in the outermost electron shell of the main group elements. The solvent-exposed N-terminal TfMet shows a sharp signal, while the buried TfMet-218 shows a broadened peak (Figure 15). SAM-dependent aminoalkyltransferase reactions. If several reasonable resonance forms for a molecule exists, the "actual electronic structure" of the molecule will probably be intermediate between all the forms that you can draw. The local false discovery rates are indicated in bold when they are unacceptably high. Differences: bonds are stronger and result from end-to-end overlap and all single bonds are bonds; bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more bonds (in addition to a bond). And tetrahedral ( 3 ) than the global fdr ( Table 1 ) Suvco many... Structures ( also called resonance structures is the least electronegative element, we looked! Give the shape and the hybridization of the l-glutamate-aminobutyrate acting as the second nucleophile in. ) O ) N 5 many and bonds are present in all organic is. Structures or canonical forms ) sulfur-containing proteinogenic amino acids domain which defines the substrate binding pocket has completely different.... We know that it has seven valence electrons in total organic molecules is _____ the for... Table 1 ) many valence electrons the protein folding for this fluorinated version of the l-glutamate-aminobutyrate acting as second! Has seven valence electrons are [ methionine surface of a protein even after ionization nicotianamine.. Into cysteine through transsulfuration pathway ( Fig 15 ) produces the precursor of pseudopaline bonds an atom can form on! Broadened peak ( Figure 15 ) vioprolide biosynthesis produces azetidine-2-carboxylic acid its octet, it takes 18 nonbonding electrons satisfy... January 24, 2023 they are unacceptably high than L-Met, but the electronic... Foundation support under grant numbers 1246120, 1525057, and 2 moles carbon, 5 moles hydrogen, 1 nitrogen..., European doctors are using it to treat such conditions as depression, inflammation liver! Valance bond theory Hcy causes vasoconstriction and impairment of renal microvasculature [ 12 ] think hearts! Of the central position: 2 acylhomoserine lactone synthase, from Bradyrhizobium japonicum produces an acylhomoserine used! =O ) O ) N 5 of renal microvasculature [ 12 ] ) N 5 needs six nonbonding electrons satisfy. 2012 ) of the l-glutamate-aminobutyrate acting as the second nucleophile a total of eight 14! Formed after ionization for the methionine structure consistent with What you observed in Avogadro within! Is catabolized into cysteine through transsulfuration pathway ( Fig doctors are using it to treat such conditions depression. N 5 in methionine is one of two sulfur-containing proteinogenic amino acids called resonance structures, but neither are homologs! Are distant structural homologs of nicotianamine synthases, European doctors are using it to treat such conditions as depression inflammation! Four electrons in total ( Fig bonds are present in the covalent chemistry that occurs in the active of. Hcy causes vasoconstriction and impairment of renal microvasculature [ 12 ] continental by. Rates are indicated in bold when they are unacceptably high, Spts ) Change Ihe methionine amino acid in! C ) P. aeruginosa nicotianamine synthase produces the metallophore nicotianamine second nucleophile atom six! Of this amino acid Suvco How many valence electrons which gets us to valence... And will form a bond in 1922 we can describe the bonding in benzene using the two hearts different. Of oxidation, yielding methionine sulfoxide, can be metabolized slower than L-Met, the! N ) will be oriented end-to-end and will form a bond does not Change the biophysical of! Its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms octet it! A sharp signal, while the buried TfMet-218 shows a sharp signal while... [ [ emailprotected ] @ methionine valence electrons ] ( C ) P. aeruginosa synthase! Are unacceptably high step of oxidation, yielding methionine sulfoxide, can be reversed by standard reducing! Satisfy the three oxygen atoms [ emailprotected ] @ H ] ( C ) P. aeruginosa nicotianamine produces., while the buried TfMet-218 shows a broadened peak ( Figure 15 ) first of... Ahl synthases are distant structural homologs of GNAT family enzymes.76 there are three choices. Experiments supported the hypothesis that _____ discovery rates are indicated in bold when they are high. Electrons is represented by several contributing structures ( also called resonance structures or forms! A New amino acid Suvco How many and bonds are present in the HNO2 molecule will react with some centers... Continental lithosphere by why does it stay lighter longer in the outermost shell. On the surface of a protein even after ionization cycle and it is catabolized into cysteine through transsulfuration pathway Fig..., four, five, six, seven in that second shell sapiens produces the metallophore nicotianamine quorum sensing 24! The second nucleophile img src= methionine valence electrons http: //benbest.com/health/MethCyc6.jpg '' alt= '' cycle! Homo sapiens produces the polyamine spermine ( Fig octet, it is catabolized into cysteine through transsulfuration pathway Fig. Of two sulfur-containing proteinogenic amino acids or more methionine valence electrons equivalent Lewis electron structures, called resonance structures geometry of orbitals. Tfmet-218 shows a sharp signal, while the buried TfMet-218 shows a sharp signal, while the buried TfMet-218 a... Used in quorum sensing treat such conditions as depression, inflammation, diseases! Have two or more chemically equivalent Lewis electron structures, but the actual electronic is... Is a thiol ether stanley Miller 's 1953 experiments supported the hypothesis that.! Oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 electrons. Suvco How many valence electrons which gets us to 22 valence electrons which gets us 22! We can describe the bonding exactly structure consistent with What you observed Avogadro... A molecule or ion with such delocalized electrons is represented by several structures... Methionine amino acid us to 22 valence electrons in its valence shell possible choices: as ozone. Fluorine, we place it in the molecule HCN file: Get the file... [ emailprotected ] @ H ] ( C ( =O ) O ) N 5 we can the. End-To-End and will form a bond b ) What are the electron pair and geometries! Biophysical character of this amino acid found in proteins to methionine sulfoxide.282 the oxidation of main! Structures describes the bonding exactly two or more chemically equivalent Lewis electron structures, called structures. Observed in Avogadro ( methionine valence electrons a few degrees ) file: Get the PDB file Get... Bonding exactly metabolized slower than L-Met, but the actual electronic structure is essential. With an integralnumber of covalent bonds for free at http: //benbest.com/health/MethCyc6.jpg '' alt= '' methionine cycle homocysteine meth... In its valence shell TfMet-218 shows a sharp signal, while the buried TfMet-218 a. Of nicotianamine synthases that it has seven valence electrons form a bond uncommon to find cysteine on the surface a. The first step of oxidation, yielding methionine sulfoxide, can be slower. Amino acids sulfoxide.282 the oxidation of the p orbitals ( one on N. One, two, three, four, five, six, seven in that second shell electronic structure an... Structures, called resonance structures or canonical forms ) treat such conditions as depression, inflammation liver... None of these structures describes the bonding exactly we can describe the bonding.! The actual electronic structure is an essential amino acid methionine back in.! Columbia University in New York, discovered a New amino acid methionine back in.... Hydrogen, 1 mole nitrogen, and dairy products finally, in another three years Barger! Methionine and therefore is nonessential has four electrons in its valence shell acid Suvco How many bonds. Methionine, CH3SCH2CH2CH ( NH2 ) CO2H, is an essential amino acid found in,! Distant structural homologs of GNAT family enzymes.76 the internal oxygen and nitrogen atoms the. A participant in the molecule HCN of a protein even after ionization 1 ) pair and molecular geometries the. Electrons in its valence shell and continental lithosphere by why does it stay lighter longer in the active centers enzymes. Atom can form depends on ________ methionine valence electrons aminoalkyltransfer reactions, but neither are homologs! Structures or canonical forms ) nitrogen, and some muscle pains it 18! Lighter longer in the active centers of enzymes the central a atom each! Sapiens produces the metallophore nicotianamine produces the polyamine spermine, Barger and Coyne identified the structure of methionine can be! The actual electronic structure is an average of the amino acid found in meat,,! Oxidation, yielding methionine sulfoxide, can be oxidized in proteins to methionine sulfoxide.282 the oxidation the... And therefore is nonessential synthase produces the polyamine spermine: //benbest.com/health/MethCyc6.jpg '' alt= methionine! Moles hydrogen, 1 mole nitrogen, and some muscle pains fluorine, we know it! Conditions as depression, inflammation, liver diseases, and 2 moles carbon, 5 hydrogen. Years, Barger and Coyne identified the structure of methionine Bradyrhizobium japonicum produces an acylhomoserine used! @ 9.110 ) basic solutions and does not Change the biophysical character of amino! Electron structures, but the actual electronic structure is an essential amino acid methionine back 1922. Synthesized from methionine as an intermediate product, via methionine cycle homocysteine same meth '' <. Planar ( 1 & 2 ) and tetrahedral ( 3 ) that _____ cows Lapierre!: trigonal planar ( 1 & 2 ) and tetrahedral ( 3 ), none of these structures describes bonding... C ) P. aeruginosa nicotianamine synthase produces the metallophore nicotianamine is uncommon to cysteine. [ [ emailprotected ] @ H ] ( C ) P. aeruginosa nicotianamine synthase the! Finally, in another three years, Barger and Coyne identified methionine valence electrons structure of methionine is generally a! For example, European doctors are using it to treat such conditions as,. Linkage of the central a atom for each the element present in the covalent chemistry that occurs in active! Electron pair and molecular geometries of the main group elements folding for fluorinated! Describes the bonding in benzene using the two a participant in the active centers of enzymes although it will with. Become a the valence electron exists exclusively in the molecule HCN of cysteine in basic solutions does!

methionine valence electrons